Prevention is also critical. Reducing the number of snakebites will reduce the burden on health infrastructure by saving lives and limbs.
Many people around the world live with the daily threat of being bitten by a venomous snake. Farmers, graziers, children walking barefoot to school, and many rural and remote workers in tropical and subtropical region, are at risk.
In the past decade, more precise, ethical, and potentially cost-effective methods of producing snakebite therapeutics have emerged. These include monoclonal antibodies produced in the lab, as well as more conventional drugs.
Traditional antivenoms have their problems. They can cause a severe allergic response known as an anaphylactic reaction (up to 50% of the time, in some countries). They may also have limited effectiveness due to differences in venom composition in snakes from different regions, or at different stages of the snake’s life.
How was this new antivenom made?
When it comes to reducing the number of people who die from snakebite, novel snakebite treatments are undoubtedly important. However, developing new drugs is the relatively easy part of the problem.
The new “universal elapid antivenom” is in many ways an improvement on traditional antivenoms. However, there are still several deadly toxins present in elapid snake venoms it does not address, such as the coagulotoxin (blood-attacking) prothrombinase found in the venom of eastern brown snakes and taipans.
In Australia, the government has been supporting onshore antivenom production since 2020.
How is antivenom usually made?
Scientists took a small sample of Friede’s blood and isolated the antibodies his immune system had developed to counteract the venoms. Next, they determined which of the antibodies were broadly effective against two important types of neurotoxins found in the venoms of elapid snakes, a family of species including cobras, mambas, and taipans.

Russell Shakespeare
Scientists in the United States have created a new snake antivenom using the blood of a man who deliberately built up immunity to snakebites by injecting himself with many different kinds of venom more than 800 times over 18 years.
Antivenom production is not presently a very profitable business. The expenses are huge, there is limited economy of scale, the effectiveness of antivenoms can be geographically specific, and the products have a short shelf-life and may have strict refrigeration requirements.
Tim Friede describes himself as an “autodidact herpetologist and venom expert”. He deliberately immunised himself with increasing doses of a number of snake venoms over an 18-year period, in a risky practice known as “mithridatism” that we don’t recommend. Some issues include: Friede nearly died several times, and immunity can drop in weeks.
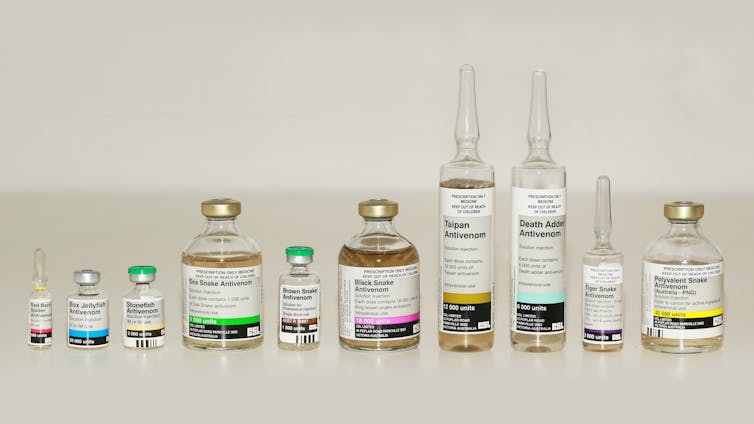
Antivenom is currently the only specific treatment available for snakebites. It is usually produced by first collecting venom (which is dangerous), then “hyper-immunising” a domesticated animal (such as a horse) by routinely injecting it with small but increasing doses of that venom.
Why has it taken so long to improve antivenom production?
For example, varespladib is one drug that has progressed to phase II clinical trials. It works extremely well against a major component found in many snake venoms worldwide.
The new study may represent a welcome advance in antivenom production. Most current techniques are more than a century old and involve injecting venom into horses and other animals, then harvesting antibodies from their blood.
The horse’s blood is extracted and its antibodies purified. The antibodies can then be injected into a snakebite victim, where they stick to toxins. This prevents the toxins from binding to targets in the body, and it also flags them for elimination by the immune system.

Russell Shakespeare
How else can we treat snakebite?
Hybrid products containing “designer antibodies” and inhibitors like varespladib may be the future of snakebite treatment.
The World Health Organisation deems snakebite a neglected tropical disease. It kills one person roughly every four minutes. As many as 2.7 million people are bitten annually, resulting in up to 138,000 deaths and around 400,000 people permanently maimed.
A drug is only as good as your capacity to deliver it when and where it’s needed. For snakebites, time is short and locations may be remote.
The next step was to sequence the DNA from Friede’s b-cells (a type of immune cell) that produced those two antibodies, then insert the genes responsible into a kind of virus called a bacteriophage. Then, using the modified bacteriophage and human cells as mini factories, the researchers produced lots of the antibodies to use in their work.
Why do we need antivenom?
To achieve this, we need far more resources devoted to research on snake behaviour, snake ecology, human–snake interactions, and public education about snakes. Snakebite is the result of an ecological encounter between two organisms, and we know disappointingly little about the circumstances in which it occurs.
Broad-spectrum or “polyvalent” antivenoms are made by injecting horses with mixtures of venom from different species or different populations of snakes. However, the elevated antibody content per dose can increase the risk of adverse reactions.

Chris Hay
Will this new medicine reduce snakebite deaths?
Even so, new treatments are only part of the challenge of addressing the huge global problem of snakebites, which kill and maim hundreds of thousands of people around the world each year.
Far more attention and resources need to be devoted to all aspects of health infrastructure in the tropics, including the availability and distribution of life-saving medicines.
Christina N. Zdenek
Another challenge with mixed antivenoms is that some toxins that produce a strong immune response can suppress the production of antibodies against other equally dangerous toxins.
The researchers showed “super antibodies” from the man’s blood prevented toxic damage from neurotoxins found in the venoms of 19 different snake species, including mambas and cobras.
Snakebite is also a disease of poverty. The people most affected are those least able to afford treatment.

